Atom
 In chemistry and physics, an atom (Greek átomos meaning "indivisible") is the smallest particle of a chemical element that retains its chemical properties. Whereas the word atom originally denoted a particle that cannot be cut into smaller particles, the atoms of modern parlance are composed of subatomic particles:
In chemistry and physics, an atom (Greek átomos meaning "indivisible") is the smallest particle of a chemical element that retains its chemical properties. Whereas the word atom originally denoted a particle that cannot be cut into smaller particles, the atoms of modern parlance are composed of subatomic particles:- electrons, which have a negative charge, a size which is so small as to be currently unmeasurable, and which are the least heavy (i.e., massive) of the three;
- protons, which have a positive charge, and are about 1836 times more massive than electrons; and
- neutrons, which have no charge, and are about 1838 times more massive than electrons.
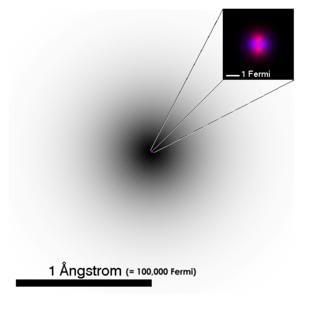
Protons and neutrons make up a dense, massive atomic nucleus, and are collectively called nucleons. The electrons form the much larger electron cloud surrounding the nucleus.
Atoms can differ in the number of each of the subatomic particles they contain. Atoms of the same element have the same number of protons (called the atomic number). Within a single element, the number of neutrons may vary, determining the isotope of that element. The number of electrons associated with an atom is most easily changed, due to the lower energy of binding of electrons. The number of protons (and neutrons) in the atomic nucleus may also change, via nuclear fusion, nuclear fission or radioactive decay, in which case the atom is no longer the same element it was.
Atoms are electrically neutral if they have an equal number of protons and electrons. Atoms which have either a deficit or a surplus of electrons are called ions. Electrons that are furthest from the nucleus may be transferred to other nearby atoms or shared between atoms. By this mechanism atoms are able to bond into molecules and other types of chemical compounds like ionic and covalent network crystals.
Atoms are the fundamental building blocks of chemistry, and are conserved in chemical reactions.
Taken from Wikipedia

0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home